News
Nature|CHIPNOVA in-situ liquid-phase electrochemistry helps to crack the "black box" and reveal a new mechanism of charge storage and aggregation reactions
2023-09-15
On September 6, Beijing time, the team of Professor Liao Honggang and Academician Sun Shigang of Xiamen University, the team of Academician Chen Jianfeng of Beijing University of Chemical Technology, and the team of researchers Xu Guiliang and Khalil Amine of Argonne National Laboratory in the United States were published in the international Ding-level journal NatureThe latest research results entitled "Visualizing Interfacial Collective Reaction Behaviour of Li-S Batteries" were published in the journal.
Based on the self-developed and established high-spatiotemporal resolution electrochemical in-situ liquid-phase transmission electron microscopy (EC-TEM), the interface reaction process of lithium-sulfur batteries has been studied in depth, and a new mechanism of charge storage and aggregation reaction of lithium-sulfur batteries has been discovered for the first time.
Corresponding authors: Prof. Honggang Liao, Prof. Guiliang Xu, Prof. Khalil Amine
First author: Zhou Shiyuan, Shi Jie

▲ Key findings: Charge storage and aggregation reaction behavior of lithium-sulfur batteries
Driven by the goal of "carbon peaking and carbon neutrality", the development of secondary battery systems with high energy density and energy storage efficiency has become a research hotspot. Uncovering the chemistry of the electrode-electrolyte interface at the atomic/molecular level is critical for battery design. The current understanding of the underlying mechanisms mainly relies on classical electrochemical theory and the Gouy-Chapman-Stern (GCS) electric double-layer model of the solid/liquid interface. In this model, the reactive species diffuses to the surface and adsorbs to undergo an electrode reaction for transformation. However, the process of electrochemical reactions that occur at the electrode meter/interface is still unclear and acts like a mysterious "black box".
However, due to the limitations of the spatiotemporal resolution of traditional in-situ characterization tools and the challenges of instability and environmental sensitivity of lithium-sulfur systems, there is still a lack of understanding of the interface reactions of lithium-sulfur batteries at the atomic/nanometer scale. To this end, the Xiamen University team used the CHIPNOVA transmission electron microscope liquid electrochemical in-situ system, coupled with the real electrolyte environment and the applied electric field, to realize the real-time observation and research of atomic-scale dynamics of the interface reaction of lithium-sulfur batteries.
In this work, the team of Prof. Honggang Liao and Academician Shigang Sun discovered that there is a unique interfacial reaction mechanism in lithium-sulfur batteries, that is, the introduction of the active center of metal nanoclusters to induce the aggregation and charge storage of lithium polysulfide (LiPSs), resulting in the transient transformation of Li2S nanocrystals from the LiPSs enrichment phase to the non-equilibrium state. This finding is different from the classical pathway in which LiPSs are gradually converted into Li2S2 and Li2S in traditional electrochemical reactions. Molecular dynamics simulations confirmed that the long-range electrostatic interaction between the active center and the LiPSs molecules led to the formation of interfacial molecular aggregates and the collective electron transfer at the electrode interface.
Video 1: Single-molecule reaction process on the surface of a blank electrode
▲Video 2: Reaction process of aggregates on the surface of the active center
This work discovers a novel interfacial reaction process for lithium-sulfur batteries. Different from the diffusion, adsorption and transformation processes of individual molecules involved in the traditional GCS model, the new mechanism of charge storage and aggregation reaction of lithium-sulfur batteries reveals the long-range interaction between metal active centers and LiPSs, the morphology of LiPSs aggregates, collective charge storage and the instantaneous crystallization of Li2S at the atomic/molecular scale. In the future, the design and development of electrode materials and systems for lithium-sulfur batteries will be promoted from a new perspective based on the new mechanism of charge storage and aggregation reaction, and the development of high-energy, high-power, and fast-charging lithium-sulfur batteries will be promoted. This collective electron transfer reaction behavior observed in lithium-sulfur battery systems has recently been observed in other electrochemical systems as well.
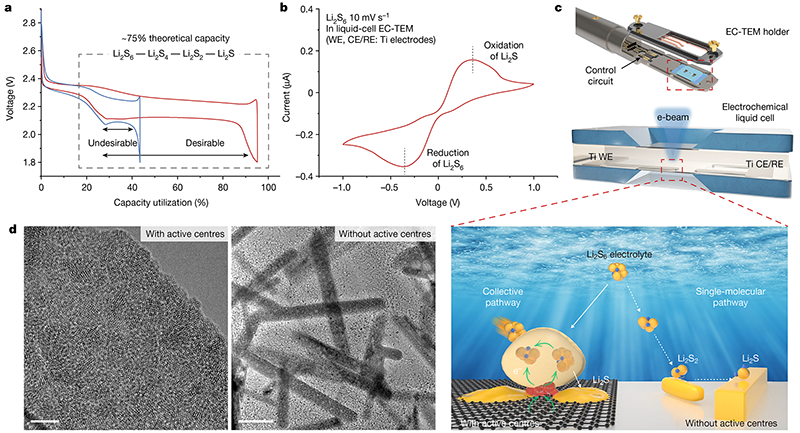
Fig.1 Design of electrochemical in-situ liquid-phase transmission electron microscope and study of the interface reaction of lithium-sulfur batteries
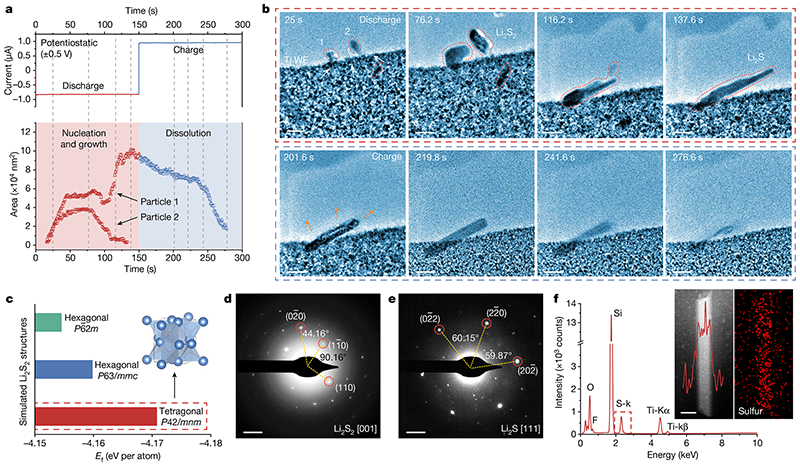
Fig.2 Single-molecule reaction process at the interface of the inactive center
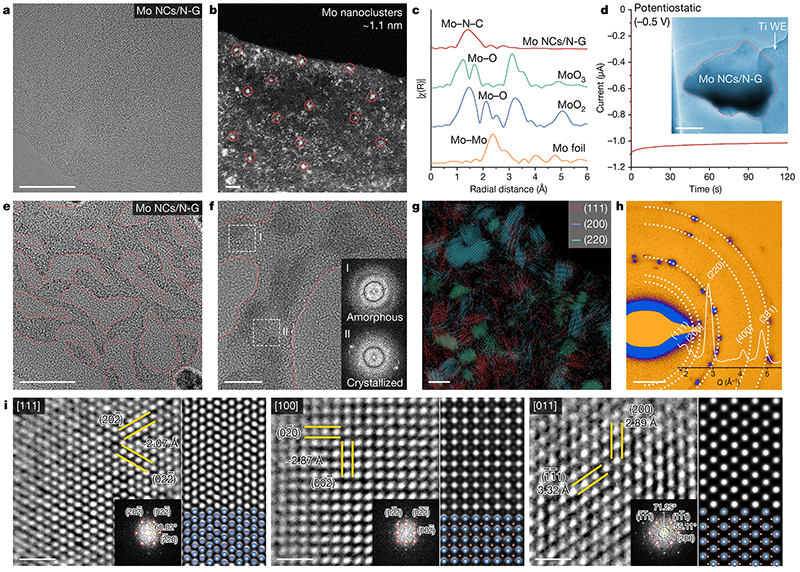
Fig.3 Lithium sulfide deposition structure at the interface of the active center

Fig. 4: Charge storage aggregation reaction at the active center interface
At the same time, the journal Nature also carried out a popular science report on the results in the form of Research Briefing.
Yohan Dall'Agnese, associate editor of the journal Nature, commented on the work – the paper is striking because the authors reveal a completely unexpected energy storage mechanism in lithium-sulfur batteries. This is an extremely rare situation, considering that this type of battery has been extensively studied for decades. These observations benefit from the development of impressive high-resolution in-situ transmission electron microscopy, and they will help improve the next generation of batteries.
Peer experts spoke highly of this work – it fills a huge knowledge gap on how to commercialize high-energy, low-cost lithium-sulfur batteries. The authors' imaging results resolve the long-standing debate about the origin and evolution of the polysulfide shuttle effect, as well as the slow dynamics of interfacial reactions in these cells, and confirm the influence of electrode surface structure on these processes. The results are of great significance for both battery and electron microscopy studies.
Related paper information
Body of paper: https://www.nature.com/articles/s41586-023-06326-8
Research Briefing:https://www.nature.com/



