News
Good news | "Discovery of a New Mechanism of Interfacial Charge Storage and Aggregation Reaction in Lithium-Sulfur Batteries" was selected as one of the "Top Ten Advances in Chinese Science" in 2023
2024-03-01
On February 29, the National Natural Science Foundation of China released the top ten scientific advances in China in 2023. The research results of Professor Liao Honggang of Xiamen University, the team of Academician Sun Shigang of Xiamen University and the team of Academician Chen Jianfeng of Beijing University of Chemical Technology, the founder of Super New Chip, "Discovery of a New Mechanism of Charge Storage and Aggregation Reaction at the Interface of Lithium-sulfur Batteries" were selected.

Professor Liao Honggang (left) attended the 2023 China Science Top Ten Progress Conference and received the award on behalf of the team
Background
The development of battery systems with high energy density and high power is an important way to achieve the goal of carbon peak and carbon neutrality. Lithium-sulfur batteries have an extremely high energy density (theoretical: 2600 Wh kg-1However, due to the spatiotemporal resolution of traditional in-situ characterization tools and the instability and environmental sensitivity of lithium-sulfur systems, the interface and reaction process of lithium-sulfur batteries at the atomic/nanoscale are not well understood, and the widespread application of lithium-sulfur batteries has not yet been realized. At present, the understanding of the energy storage mechanism is mainly based on the electric double-layer model of the solid/liquid interface and the classical electrochemical theory, in which the reactant molecules diffuse to the electrode surface and undergo electron transfer and desorption away from the surface. In the study of chemical reactions and charge transfer, Henry Taube won the Nobel Prize in 1983 for his contribution to the discovery of the "inner ball reaction" mechanism of electron transfer, and Rudolph A. Marcus won the Nobel Prize in 1992 for his contribution to the "inner ball reaction" mechanism of electrochemical reaction, and later extended to the inner ball reaction mechanism, basically unifying the electrochemical electron transfer reaction theory. However, due to the limitations of the spatiotemporal resolution of traditional characterization tools, their theories are based on a simple single-molecule model to understand the electron gain and loss reactions. The reactive molecule gets electrons on the surface of the electrode, and the configuration transformation occurs, and the coordination environment around the molecule changes accordingly to reach a new stable state, and the reactive molecule becomes a new substance through the single-molecule electron gain and loss reaction pathway. However, there are thousands of molecules, ions, etc. at the real electrode surface/interface, and the electrochemical reaction process that occurs is still unclear, like a mysterious "black box".
Research Results
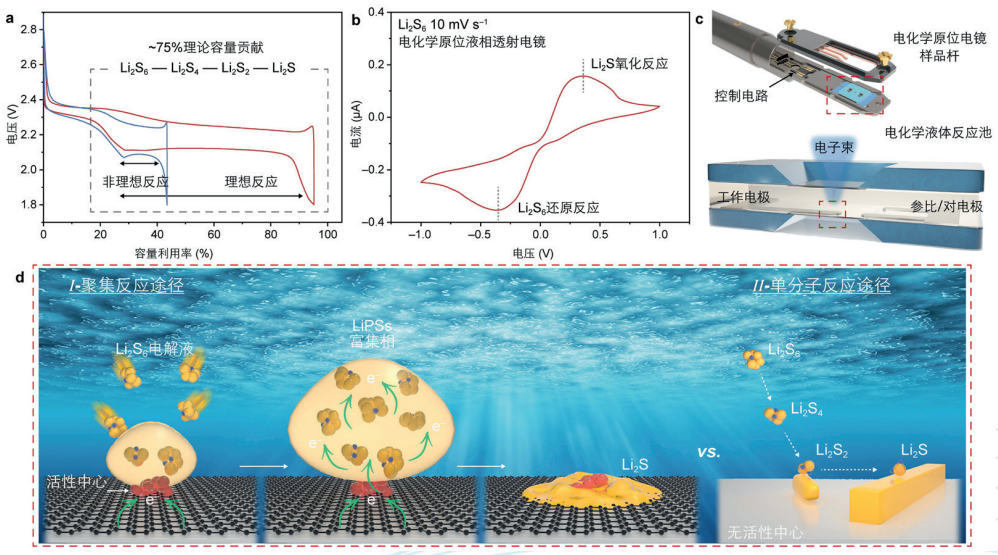
Electrochemical in-situ transmission electron microscopy was used to study the interfacial reactions of lithium-sulfur batteries
Independently developed high-spatiotemporal resolution electrochemical in-situ liquid-phase transmission electron microscopy technology, coupled with the real electrolyte environment and the applied electric field, real-time observation and research of the interface interface and reaction process of lithium-sulfur batteries at the atomic scale were realized, and a new mechanism of charge storage and aggregation reaction at the interface of lithium-sulfur batteries was discovered, that is, on the surface of the material with the active center of the battery, the lithium polysulfide molecules gathered into molecular clusters at the active center for reaction: the transferred charge was first stored in the aggregated molecular clusters, and the electrons obtained by the molecular clusters did not immediately transform. Until sufficient electrons are obtained, it is transiently crystallized into amorphous lithium sulfide. On the surface of materials without active centers, the classical single-molecule reaction pathway is followed: a single lithium polysulfide molecule receives electrons, is gradually converted, and finally converted into Li2S molecules, which are subsequently crystallized and grown.
Significant
In the past 100 years, electrochemical interfacial reactions have generally been thought to exist only in the "endosphere" and "outer sphere" single-molecule pathways. The discovery of this new mechanism reveals that there is a "charge storage aggregation reaction" mechanism in electrochemical interface reactions in addition to the traditional single-molecule reaction pathway. This study deepens the understanding of polysulfide evolution and its impact on the reaction kinetics of the battery surface interface, and provides guidance for the design of next-generation lithium-sulfur batteries. The related research paper was published in Nature [621(7977): 75-81] on September 7, 2023. Since the concept of lithium-sulfur batteries was proposed in the sixties of the last century, this study is the first international research paper on lithium-sulfur batteries to be published in the journal Nature. Yohan Dall Agnese, associate editor of the journal Nature, commented on the work: "It is striking that the authors have revealed a completely unexpected energy storage mechanism in lithium-sulfur batteries, which has been extensively studied for decades, which is extremely rare. The development of high-resolution in-situ electron microscopy is impressive, and the discovery of new technology developments and new mechanisms will greatly assist in the design of next-generation batteries. ”
Profile

Prof. Honggang Liao
Professor, doctoral supervisor, national high-level introduced talents, Minjiang scholars distinguished professor. His main research areas are the development of in-situ electron microscopy and its application in material synthesis, electrocatalysis, energy storage and conversion processes. He has completed the early demonstration research of in-situ liquid transmission electron microscopy, published more than 100 papers in Science, Nature and its sub-journals, JACS, Angew, EES and other journals, applied for and authorized more than 60 patents and soft works, and independently developed a variety of in-situ electron microscope chips and analysis systems, which can be widely used in basic research and industrial upgrading. At present, he is a member of the Expert Committee of the Chinese Energy Society, a member of the Professional Committee of Polymer Material Analysis Technology and Characterization Methods of the Chinese Chemical Society, the chairman of the Supervisory Board of the Entrepreneurship Branch of the Xiamen University Alumni Association, and the general manager of Xiamen Chaoxinxin Technology Co., Ltd.
A variety of in-situ electron microscope chip reactors and systems developed by him introduce liquids and gases into the electron microscope and combine them with electricity, heat, light, force and other external fields to realize real-time imaging at the atomic scale, obtain information on dynamic reaction processes such as valence states, and provide a new microscopic perspective for the basic research and application of chemistry and materials. The real-time observation and study of the nucleation growth and morphological evolution of a variety of nanocrystals in solution has revolutionized the understanding of the growth law of nanocrystals, which has been reported as "shaping the future of nanocrystals" and "subverting the cognition of crystal growth laws for more than 100 years". In situ studies of electrochemical reactions at the atomic/molecular scale reveal the aggregation patterns of molecules and ions on the electrode surface, as well as the electron transfer reaction process, and find that there is a third mechanism of "charge storage aggregation reaction" in electrochemical interface reactions, which provides guidance for the design of next-generation batteries.
The main product of the scientific instrument sector of the industrialization company, the electron microscope in-situ microscopic analysis system, covers five series of liquid, gas, mechanics, heating and freezing, and its customers cover Peking University, Tsinghua University, Zhejiang University and other well-known domestic universities, Chinese Academy of Sciences and other domestic and foreign scientific research units, and various national key laboratories, breaking the monopoly of European and American companies, realizing import substitution and leading market sales.
Transmission electron microscopy liquid electrochemical in-situ system
The transmission electron microscope liquid electrochemical in-situ system uses MEMS microfabrication process to construct a liquid atmosphere nano laboratory in the in-situ sample stage, and applies electrical signals to the thin layer or nano battery system through the MEMS chip, and combines EDS, EELS, SAED, HRTEM, STEM and other different modes to realize real-time and dynamic monitoring of the microstructure evolution, reaction kinetics, phase transition, and other modes of electrode, electrolyte and their interface under working conditions from the nanometer and even atomic level. Critical information such as elemental valence, chemical changes, microscopic stresses, and atomic-level structure and compositional evolution at the surface/interface.

(Click on the image to see more product details)
Our advantages
The highest resolution in the industry
2. The chip package adopts the double insurance method of bonding inner seal and epoxy resin outer seal, so that the thinnest sandwich between the chips is only about 100~200nm, and the ultra-thin sandwich greatly reduces the interference of the electron beam, and the atomic arrangement of the sample can be clearly observed, and the atomic-level resolution can be achieved in the liquid phase environment.
3. The specially designed chip window shape can avoid the silicon nitride film bulging and causing the liquid layer to thicken and affect the resolution.
Highest security
2. The patented nanofluidic technology is adopted, and the fluid differential control is carried out through the piezoelectric microcontrol system to realize the nanoscale microfluid transportation, and the redundant liquid volume in the in-situ nanofluidic system and the sample holder is only microliter, which effectively ensures the safety of the electron microscope.
3. The polymer membrane surface contact sealing technology is adopted, which increases the sealing contact area and effectively reduces the risk of leakage compared with the O-ring sealing.
4. Using ultra-high temperature coating technology, the silicon nitride film in the chip window area has the advantages of high temperature resistance, low stress resistance, pressure resistance, corrosion resistance, and radiation resistance.
Unique multi-field coupling technology


